An Aromatic Compound Must Contain Which of the Following
Results from the measurements were used to estimate benzoapyrene equivalent toxicity BaPeq of individual compounds in order. One common method is based on the specific elements present.
4 12 Heterocyclic Aromatic Compounds Chemistry Libretexts
These atoms can form a delocalized system of π molecular orbitals.
. Ar-NH2 Ar-N2 a process known as diazotization and step 2 is the reaction of the diazo compound with a phenol naphthol aromatic amine or a compound that has an active methylene group to produce the corresponding azo dye a process known as diazo coupling eg. Some foods contain ingredients and chemicals that are harmful in large amounts. Aromatic hydrocarbons contain the 6-membered benzene ring structure A that is characterized by alternating double bonds.
Example Benzene and pyrrole are aromatic in nature while acyclic compound C 4 H 5 NH 2 is a non-aromatic compound. Aromaticity of an arbitrary aromatic compound can be measured quantitatively by the nucleus-independent chemical shift. Step 1 is the conversion of an aromatic amine to a diazo compound ie.
Ar-N2 Ar-OH Ar-NN-Ar. Here are 7 food toxins that are actually concerning. This involves heating a C6-C10 alkane fraction of petroleum with hydrogen in the presence of a.
1 The structure must be cyclic and contain some number of conjugated bonds. A polycyclic aromatic hydrocarbon PAH is a hydrocarbona chemical compound containing only carbon and hydrogenthat is composed of multiple aromatic ringsThe group is a major subset of the aromatic hydrocarbonsThe simplest of such chemicals are naphthalene having two aromatic rings and the three-ring compounds anthracene and phenanthrene. Learn how to write ionic compound formulas for binary and polyatomic compounds and.
Simple aldehydes and ketones are named using the standard rules of nomenclature which we have used in the past with the following specific changes. Organic compounds are characterized as those compounds with a. Aromatic compounds are synthesized from petroleum the by a process referred to in the petroleum industry as catalytic re-forming or hydroforming.
For example oxides contain one or more oxygen atoms hydrides contain one or more hydrogen atoms and halides contain one or more halogen Group 17 atoms. Chemical compounds may be classified according to several different criteria. The repeating monomer of Ultradur is shown in B.
In pyrrole each of the four sp 2-hybridized carbons contributes one π-electron and the nitrogen atom is also sp 2-hybridized and contributes two π-electrons from its. Aldehydes are named by replacing the terminal -e of the parent alkane with the suffix -al. The suffix for ketones is -one.
The parent chain selected must contain the carbonyl group. An aromatic molecule must be cyclic. Compounds must fulfill the following four conditions to be an aromatic compound The molecule must be cyclic.
An aromatic compound ring should consist of only sp 2-hybridized atoms. For example Nitro group increases the electronic deficit of the diazonium group. If a compound or a molecule meets the following criteria then these are aromatic compounds.
Structures are given below image Every atom in the cyclic ring must be conjugated. Five unsubstituted PAHs were included for comparison. Ionic compounds both binary and polyatomic contain positively-charged cations and negatively-charged anions.
The coupling reaction can take place when an alkyl-substituted aromatic derivative which is the nucleophilic reagent in which case the electrophile must contain an electron withdrawing substituent. 22 alkylated polycyclic aromatic hydrocarbons alk-PAHs were characterized in ambient air individually for the first time in urban and semi-urban locations in Toronto Canada. Ultradur PBT is a plastic polymer that contains an aromatic functional group.
As it will provide the cyclic ring delocalized pi. Pyrrole and imidazole are both five membered aromatic rings that contain heteroatoms. An aromatic molecule must be planar.
This allows the coupling of 2 4 6-trinitroaniline with 1 3 5-trimethylbenzene. In the delocalized π system the number of π electrons must be equal to. Ultradur can be found in showerheads toothbrush bristles plastic housing for fiber-optics cables and in automobile.
The number of pi electrons must be equal to 4 n 2 where n is equal to zero or any positive integer n. Although petroleum does contain some aromatic compound it primarily made up of alkanes of various chain lengths. Ch16 Aromatic Compounds landscapedocx Page 19 Aromatic Antiaromatic and Nonaromatic Compounds In a more specific chemical sense aromatic compounds are defined as those which meet the following criteria.
Huckels Rule states that an aromatic compound must have a certain number of pi electrons.

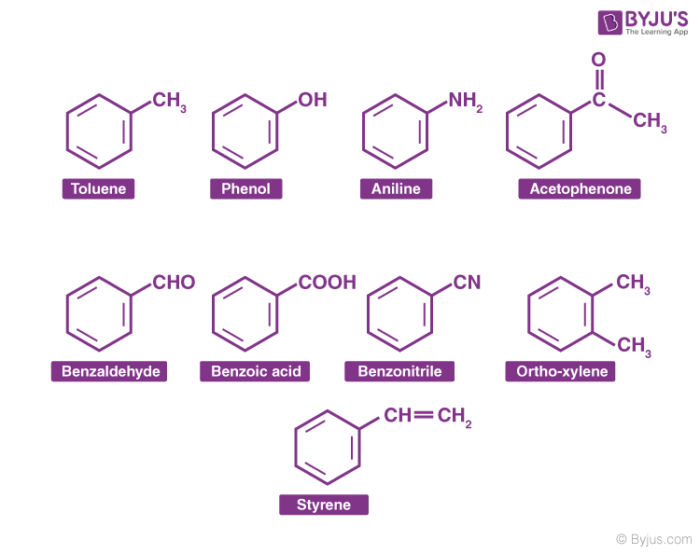
Naming Aromatic Compounds Chemistry Steps
Aromatic Compounds Definition Example Properties Nomenclature With Videos

What Is An Aromatic Compound Definition Example Video Lesson Transcript Study Com
No comments for "An Aromatic Compound Must Contain Which of the Following"
Post a Comment